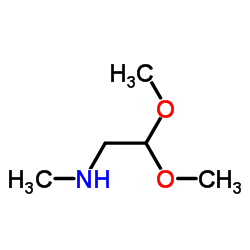
Chemical Name: Methylaminoacetaldehyde dimethyl acetal
CAS.NO:122-07-6
Molecular Formula:C5H13NO2
Molecular Weight:119.16200
Synonyms:
2,2-Dimethoxy-N-methylethylamine
2,2-dimethoxy-N-methylethanamine
Thiamazole impurity A
1,1-Dimethoxy-2-methylaminoethane
N-(2,2-Dimethoxyethyl)methylamine
Ethanamine, 2,2-dimethoxy-N-methyl-
Methylaminoacetaldehyde Dimethyl Acetal
(Methylamino)acetaldehyde dimethyl acetal
Physical and Chemical Properties:
Density: 0.928 g / mL at 25 ° C (lit.)
Boiling point: 140 ° C (lit.)
Melting point: -73 ° C
Flash point: 85 ° F
Refractive index: n20 / D 1.414 (lit.)
Specification:
Appearance: Yellow powder
Purity:≥99.0%
Total Impurity:≤0.5%
Water:≤0.5%
Heavy metals:≤10ppm
Volatile:≤0.1%
Inorganic salt:≤0.1%
Packing:25 kg/drum, can also be packaged according to customer requirements
Storage:Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Application:2,2-Dimethoxy-N-methylethanamine is an impurity formed in the synthetic process of Methimazole, a thiourea antithyroid agent that prevents iodine organification,
We are professional Methylaminoacetaldehyde dimethyl acetal manufacturer and (Methylamino)acetaldehyde dimethyl acetal supplier in China, We offer quality Thiamazole impurity A you can fully trust, also we have India factory and producer of Ethanamine, 2,2-dimethoxy-N-methyl-,Pls send inquiry of Ethanamine, 2,2-dimethoxy-N-methyl- CAS:122-07-6 to info@nbinno.com if you have any interests, thank you!
Related News: Higher-risk MDS is a disease with significant unmet need, and we are pleased to be able to support healthcare professionals seeking access to rigosertib, ahead of its commercial launch,” said Mark Corbett, EVP, Inceptua Medicines Access.Methyl [(dimethoxyphosphinothioyl)thio]acetate Higher-risk MDS is a disease with significant unmet need, and we are pleased to be able to support healthcare professionals seeking access to rigosertib, ahead of its commercial launch,” said Mark Corbett, EVP, Inceptua Medicines Access.115118-68-8 High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.459-57-4 High-barrier generic drugs: Usually for the production of drugs whose patents have just expired or are about to expire, the market is only competed by original research and a few generic drug companies.This time frame optimizes the opportunity to respond to treatment with an HMA prior to declaring treatment failure, as per NCCN Guidelines. Patients are randomized at a 2:1 ratio into two study arms: IV rigosertib plus Best Supportive Care versus Physician’s Choice plus Best Supportive Care.


