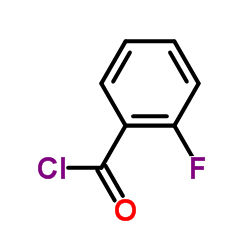
Synonyms:
2-Fluorobenzoyl chloride
o-fluoro-benzoylchlorid
2-Fluorobenzoyl
2-fluoro-benzoylchlorid
o-Fluorobenzoyl
O-FLUOROBENZOYL CHLORIDE
2-fluoro-benzoyl chloride
2-Fluorobenzoyl chlo
ortho-fluorobenzoyl chloride
2-Fluorbenzoylchlorid
2-fluorobenzoic acid chloride
Benzoyl chloride, 2-fluoro-
molecular formula: C7H4ClFO
Molecular weight: 158.55700
Physical Properties:
Appearance and Properties: Transparent to pale yellow liquid
Density: 1.328 g/mL at 25 °C(lit.)
Boiling point: 90-92 °C15 mm Hg(lit.)
Melting point: 4 °C (lit.)
Flash point: 180 °F
Refractive index: n20/D 1.536(lit.)
Water Solubility: Decomposition
Stability: Stable under normal temperatures and pressures.
Specification:
Appearance:Colorless or pale yellow, transparent liquid
Flufluenzyl chloride, w /%:≥ 99.00
Application:
Flufluoryl chloride can be used as a dye, medicine and pesticide intermediate.
Package and Storage:
This product is packed with 200L iron barrels lined with PVF cover with a net weight of 180kg.The bucket is filled with nitrogen and gas protection, and the packaging bucket is tightly sealed.
Storage: The product shall be strictly sealed and stored in a cool, dry and well-ventilated environment, far away from fire and heat source.Store separately with acid, alkali, and oxidant.Storage period for 12 months under the specified transportation and storage conditions.
We are professional 2-Fluorobenzoyl chloride manufacturer and Benzoyl chloride, 2-fluoro- supplier in China, We offer quality 2-fluorobenzoic acid chloride you can fully trust, also we have India factory and producer of ortho-fluorobenzoyl chloride,Pls send inquiry of ortho-fluorobenzoyl chloride CAS:393-52-2 to info@nbinno.com if you have any interests, thank you!
Related News: Regulators approved the shot in May but paused the deployment amid lingering concerns about blood clots. Japan has so far relied vaccines made by Pfizer Inc (PFE.N) and Moderna Inc (MRNA.O) in its inoculation push.(methylene-3′ dimethyl-5′,5′ cyclohexene-1′ yl) thio-3 cyclohexanone manufacturer He also mentioned that derivatives of these APIs and lot of other value-added APIs in the same range will also be manufactured at the new facility. 1-(3-chloro-4-hydroxy-5-(3-(2-(trifluoromethyl)phenyl)thioureido)phenyl)-3-cyclohexylurea supplier Professor Xiangdong Cheng, secretary of party committee of Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) and Chief of Zhejiang Province Upper Gastrointestinal Tumor Diagnosis and Treatment Technology Research Center, stated: The progress of immunotherapy in certain types of colorectal cancer and gastric cancer has not been satisfactory compared with the progress of anti-PD-1 monoclonal antibodies in melanoma, lung cancer and esophageal cancer, and this needs further exploration.
TIGIT is highly expressed in gastric cancer. And pre-clinical experiments have proved that synergistically targeting PD-1 and TIGIT could significantly repress gastric tumor growth. We look forward to the clinical results of IBI321, particularly in GI tumors.
Dr. Hui Zhou, Senior Vice President of Clinical Development of Innovent, stated: “By specifically targeting both PD-1 and TIGIT, this type of bispecific antibody is designed to provide synergistic effects by blocking both the PD-1/PD-L1 and TIGIT/CD155 pathways – with the goal of improving the anti-cancer efficacy.
Currently, there is no bispecific antibody that has same target as IBI321 in clinical-stage development worldwide. Preclinical studies of IBI321 have demonstrated that the combination of these two monoclonal antibodies further enhanced the immune activation with improved convenience of administration.
Therefore, the development of an anti-PD-1/TIGIT bispecific antibody has the potential to provide patients with more effective and convenient treatment. We look forward to hope that IBI321 will benefit more patients. (1S,3R)-1-Butyl-2-methylsulfanylthiocarbonyl-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid manufacturer Operating more than 80 primary care medical practices for diverse populations of seniors, ChenMed results consistently include up to 75 percent drops in emergency room visits, plus 30 to 50 percent reductions for in-patient hospital admissions. A Fortune 2020 Change the World” company, ChenMed brands include Chen Senior Medical Center, Dedicated Senior Medical Center and JenCare Senior Medical Center.
Medallia (NYSE: MDLA) is the pioneer and market leader in Experience Management. Medallia’s award-winning SaaS platform, the Medallia Experience Cloud, leads the market in the understanding and management of experience for customers, employees and citizens.
Medallia captures experience signals created on daily journeys in person, digital and IoT interactions and applies proprietary AI technology to reveal personalized and predictive insights that can drive action with tremendous business results. Using Medallia Experience Cloud, customers can reduce churn, turn detractors into promoters and buyers, and create in-the-moment cross-sell and up-sell opportunities, providing clear and potent returns on investment
QYNAPSE Inc., a medical technology company commercializing an AI-powered and proprietary neuroimaging software platform, is attending the Alzheimer’s Association International Conference 2021 (AAIC) on July 26-30, 2021 in Denver, USA and online, and will present two poster presentations on QyScore®, it’s groundbreaking neuroimaging analysis platform for both clinical routine and clinical trial settings.
Powered by the robust technology and designed to seamlessly integrate with existing workflows, QyScore® which is both FDA-cleared and CE-marked, provides insightful layers of precision for the analysis of brain MRIs, offering the potential to reduce the time to diagnosis and more precisely measure disease progression for Alzheimer’s and other central nervous system (CNS) diseases.
“We are very pleased to attend AAIC in-person in Denver this year to present and discuss our latest advances with the neuroscience community. We have made tremendous progress in 2021 towards expanding the commercialization of QyScore® into new global markets and to strengthen our clinical trial offering.
Qynapse is dedicated to bring ground-breaking peace of mind to the global fight against Alzheimer’s disease, by continuing to support clinicians around the world and build new tools that will support the ongoing care of patients in light of new disease-modifying treatment options for patients.” said Matt Ullum, CPA, MBA, Chief Commercial Officer at Qynapse.
Rapid reduction and sustained normalization of heparan sulfate in CSF demonstrated robust and durable CNS activity with intravenous administration, and enhanced peripheral activity with reductions in urine and serum heparan sulfate after switching from standard-of-care idursulfase
Global Impression of Change scales data suggested clinical improvement in overall MPS II symptoms, cognitive abilities, behavior, and physical abilities
Exploratory biomarker data demonstrated reductions in CSF lysosomal lipid biomarkers that are consistent with improved lysosomal function; and high variability in exploratory biomarker Nf-L was observed pre- and post-treatment
Safety profile with up to 43 weeks of dosing was consistent with standard of care enzyme replacement therapy with infusion-related reactions being the most frequently observed adverse events
Based on these data, Denali is accelerating efforts to initiate a pivotal Phase 2/3 study in 1H 2022
SOUTH SAN FRANCISCO, Calif., July 25, 2021 (GLOBE NEWSWIRE) — Denali Therapeutics Inc. (NASDAQ: DNLI), a biopharmaceutical company developing a broad portfolio of product candidates engineered to cross the blood-brain barrier (BBB) for neurodegenerative diseases, today announced additional positive interim data from a Phase 1/2 study evaluating ETV:IDS (DNL310), an investigational brain-penetrant enzyme replacement therapy intended to treat both central nervous system (CNS) and peripheral manifestations of Hunter syndrome (MPS II).
The interim results being presented today at MPS 2021, the 16th International Symposium on MPS and Related Diseases, include safety data up to Weeks 43 and 25 from Cohorts A and B, respectively, 6-month biomarker data from Cohort A and up to 3-month biomarker data from Cohort B. Denali Management will host a webinar today for analysts and investors beginning at approximately 11:30 a.m. Eastern Time.
“The longer-term safety data and 6-month biomarker data on DNL310 from Cohort A continue to demonstrate durability of effect with CNS impact, improved peripheral activity after switching from standard of care, and a safety profile consistent with standard of care enzyme replacement therapy,” said Carole Ho, M.D., Denali’s Chief Medical Officer.
“We are also encouraged by initial indications of improved clinical symptoms and function reported by investigators and parents in all five patients enrolled in Cohort A. In addition, this is the first time we are sharing data from Cohort B, which is designed to inform dose selection, and exploratory biomarker data demonstrate activity of DNL310 across all dose regimens.
Based on these data, we are accelerating our efforts to initiate a pivotal Phase 2/3 study of DNL310 in the first half of 2022 and to begin enrolling Cohort C in the Phase 1/2 study to further investigate clinical endpoints.”
This interim analysis of the Phase 1/2 study included data on five patients enrolled in Cohort A and 12 patients enrolled in Cohort B. All patients have neuronopathic MPS II disease except for one patient with non-neuronopathic MPS II disease in Cohort B.
The median age of patients is 6 years in both cohorts, with the youngest patients aged 5 and 2 in Cohorts A and B, respectively. All patients received weekly intravenous doses of DNL310 after switching from idursulfase enzyme replacement therapy on Day 1 of the study.
Data being presented include safety data up to Weeks 43 and 25 from Cohorts A and B, respectively; 6-month and up to 3-month biomarker data from Cohorts A and B, respectively; and exploratory clinical Global Impression of Change data from Cohort A up to Week 24.
Results across Cohorts A and B showed that, following the switch from idursulfase to DNL310, the levels of heparan sulfate in cerebrospinal fluid (CSF) normalized in all patients analyzed (n=15), with rapid response observed in most patients (n=12) by Week 7, which is consistent with crossing of the BBB by DNL310 and activity in tissues of the CNS.
Rapid normalization of CSF heparan sulfate at low dose regimens suggest that BBB crossing with Denali’s Transport Vehicle (TV) was robust and efficient. Furthermore, the observed decline in urine and serum heparan sulfate was consistent with improved peripheral activity with DNL310.
Exploratory clinical data suggest improved clinical symptoms and function for all five patients enrolled in Cohort A as reported by investigators and parents. Based on Global Impression of Change scales [Clinician Global Impression of Change (CGI-C) and Parent Global Impression of Change (PGI-C)], which are standardized assessment scales used to measure change, the data showed clinical improvement in overall MPS II symptoms, cognitive abilities, behavior, and physical abilities.
Exploratory lysosomal lipid data showed reductions, which are consistent with improved lysosomal function: 10 of 15 patients across Cohorts A and B had normal GM3 ganglioside levels, including patients on low dose regimens and with shorter duration of treatment. In addition, reductions in levels of bis(monoacyl glycerol)-phosphate (BMP) and a potential reduction in levels of glucosylceramide (GlcCer) were observed in Cohort A at Week 24.
High within patient variability in levels of neurofilament (Nf-L), an exploratory biomarker of neuronal structure, was observed pre- and post-treatment. Data from an ongoing observational natural history study conducted by Denali showed a marked increase in mean levels of serum Nf-L over a 4.5- to 6-month period in patients (n=3) who subsequently enrolled in Cohort A of the Phase 1/2 study.
During the 6-month treatment period of the Phase 1/2 study, mean levels of serum and CSF Nf-L in Cohort A (n=5) showed a modest increase. Denali believes that the utility of Nf-L as a treatment response biomarker in MPS II will require further investigation.
The safety profile of DNL310 remained consistent with standard of care enzyme replacement therapy. DNL310 was generally well tolerated with the most common treatment-emergent adverse events being infusion-related reactions (IRRs). IRRs occurred in 12 of 17 (71%) patients: the majority had mild (n=5) or moderate (n=6) IRRs, and 1 patient had severe IRRs.
A total of 3 serious adverse events (SAEs) were reported: 1 previously reported SAE for a patient enrolled in Cohort A based on a mild IRR, and 2 SAEs in a patient enrolled in Cohort B based on severe IRRs. The SAEs resolved, and both patients are continuing in the study. All other treatment-emergent adverse events were mild or moderate.
The study continues without modification following recommendation by an independent data monitoring committee on July 9, 2021.
“DNL310 is our lead program enabled by our blood-brain barrier Transport Vehicle platform, and these data continue to validate the platform’s potential as we advance additional TV-enabled programs toward the clinic,” said Ryan Watts, Ph.D., Denali’s Chief Executive Officer.
“Our DNL310 program exemplifies application of Denali’s core scientific principles to increase likelihood of success by targeting degenogenes, engineering therapeutics to cross the blood-brain barrier, and using biomarkers to inform development. We are encouraged by these interim data and we look forward to continued collaboration with the community to advance MPS II research and DNL310 as a potential treatment for affected individuals and their families.”
Families interested in learning more about Denali’s efforts related to the discovery and development of therapeutics for the potential treatment of Hunter syndrome are invited to visit EngageHunter.com, the Denali Hunter syndrome community engagement website.
Denali will host a webinar for analysts and investors to present the interim data from the Phase 1/2 study of DNL310. The webinar will begin at approximately 11:30 a.m. EDT / 8:30 a.m. PDT on Sunday, July 25, 2021, and will be available on Denali’s corporate website on the Events page under the Investor section and can be accessed by following this link.
SAN FRANCISCO and SUZHOU, China, July 25, 2021 /PRNewswire/ — Innovent Biologics, Inc. (Innovent) (HKEX: 01801), a world-class biopharmaceutical company that develops, manufactures and commercializes high-quality medicines for the treatment of cancer, metabolic, autoimmune and other major diseases, today announced that the first patient has been dosed in a Phase 1 study of IBI321, an anti-PD-1/TIGIT bispecific antibody.
The objective of this open-label, multi-center Phase 1a dose escalation and expansion study is to evaluate the safety, tolerability, potential optimal dosage and preliminary efficacy of IBI321 in patients with advanced malignant tumors whose cancer progressed on standard-of-care treatment. The trial is being conducted in China.
As a bispecific antibody, IBI321 has the potential to enhance efficacy by synergistically targeting both PD-1 and TIGIT. Preclinical studies of IBI321 have shown that the molecule retained the activity of a PD-1 inhibitor and simultaneously suppressed TIGIT to enhance the anti-cancer efficacy.
Professor Baohui Han, Chief of Respiration Department and GCP center of Shanghai Chest Hospital, stated: “Although immune checkpoint inhibitors have shown great efficacy in a variety of tumor types, we still face challenges of primary and secondary drug resistance.
Meanwhile, the efficacy of immune checkpoint inhibitors needs to be further improved. Therefore, the development of the next generation of bispecific antibodies has important clinical value. TIGIT is an important immune checkpoint receptor which plays an important role in tumor immune surveillance. We look forward to the clinical research results of IBI321.