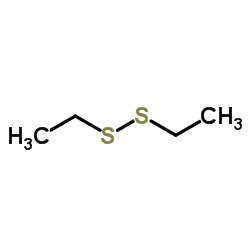
We are professional Diethyl disulfide manufacturer and Diethyl Disulfide supplier in China, We offer quality Diethyl disulfide you can fully trust, also we have India factory and producer of Disulfide, diethyl,Pls send inquiry of Disulfide, diethyl CAS:110-81-6 to info@nbinno.com if you have any interests, thank you!
Related News: Beta Bionics is committed to obtaining regulatory approval and commercializing all three iLet configurations.(Diacetoxyiodo)benzene Beta Bionics is committed to obtaining regulatory approval and commercializing all three iLet configurations.1-Bromo-2-fluoro-4-(trifluoromethoxy)benzene CAS:168971-68-4 The Company’s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).6-amino-4-[3-chloro-4-(pyridin-2-ylmethoxy)anilino]-7-ethoxyquinoline-3-carbonitrile Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.Under the terms of this agreement, Inceptua will support Onconova through the pre-approval provision of intravenous rigosertib initially into a number of countries including: Australia, Denmark, Finland, France, Ireland, Italy, the Netherlands, Portugal, South Africa, Spain, and the UK.


