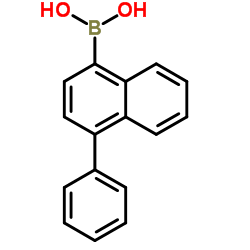
We are professional (4-Phenylnaphthalen-1-yl)boronic acid manufacturer and 4-Phenylnaphthalene-1-boronic Acid supplier in China, We offer quality 4-Phenyl(napthalene-1-yl)boronic acid you can fully trust, also we have India factory and producer of 4-Phenylnaphthalene-1-boronic Acid,Pls send inquiry of (4-phenylnaphthalen-1-yl)boronic acid CAS:372521-91-0 to info@nbinno.com if you have any interests, thank you!
Related News: Onconova Therapeutics, Inc. is a Phase 3-stage biopharmaceutical company focused on discovering and developing novel small molecule drug candidates to treat cancer, with an initial focus on Myelodysplastic Syndromes (MDS).2-Chloro-4,5-difluorobenzaldehyde Active Pharmaceutical Ingredients (APIs): Pharmaceutical active ingredients, which are the basic substances that constitute the pharmacological effects of pharmaceuticals, and are prepared by chemical synthesis, plant extraction, or biotechnology.2-Chloroanisole However, pharmaceutical intermediates are subdivided into primary intermediates and advanced intermediates. Because primary intermediate suppliers can only provide simple intermediate production, they are at the front end of the industrial chain. The pressure of competition and price is the greatest. The price fluctuations of basic chemical raw materials have a greater impact on them.ALLYL ISOCYANATE The drug review center will establish a registration platform and database for APIs, pharmaceutical excipients and pharmaceutical packaging materials, which means that the domestic drug substance DMF system is expected to be gradually implemented.Active pharmaceutical ingredients directly impact disease.


